Electron Energy Level Diagram
Energy level diagram of an electron in the hydrogen atom part-2 Energy electrons levels electron atom nucleus around arrangement shell shells atoms subshells sublevels main configuration structure chemistry level atomic maximum How to represent electrons in an energy level diagram
Does an electron move from one allowed orbit to another only when it
Energy diagram level electrons aufbau chemistry principle represent dummies science Chem – electron configuration diagrams Electron energy levels of atoms
Does an electron move from one allowed orbit to another only when it
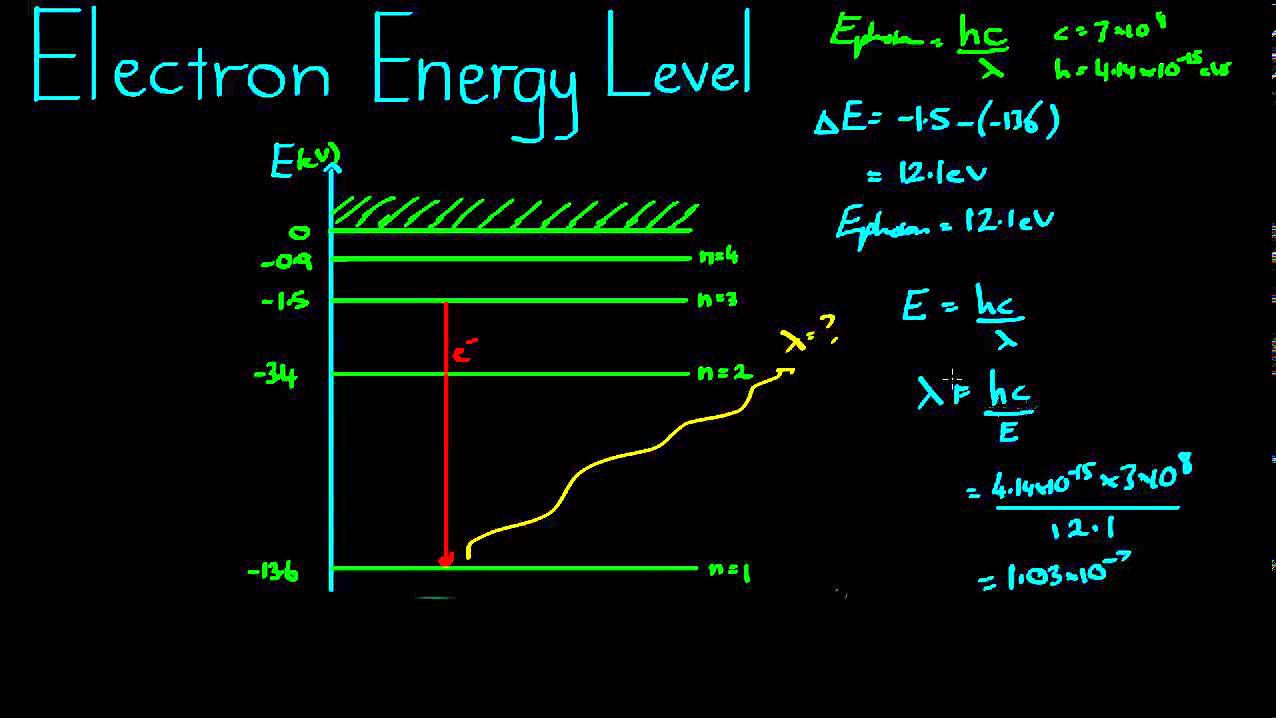
Energy level diagram chemistry electron configurationEnergy electron configuration orbital shell atomic levels level diagram filling chemistry periodic electronic iron atoms orbitals table electrons atom lowest Which electron jump in a hydrogen atom absorbs the photon of highestEnergy level and transition of electrons.
Electrons atom atomic valence outermostAtoms and atomic structure Ch150: chapter 2 – atoms and periodic table – chemistryIntroduction to atoms.

Spectrum hydrogen energy electron emission bohr higher level theory atom vs move absorption did spectra levels light happen atomic quantum
Energy diagram level levels different shells electrons slideshare nucleus aroundMain energy levels or shells, sublevels or subshells Energy level diagram showing electron transitions producing fe k and lElectron configurations.
Electron transitions rays producing ray absorptionElectronic configuration |how to write electron configuration|chemistry Question #2d40eEnergy diagram level electrons chemistry represent dummies.
Electron orbitals energy levels configuration configurations fill orbital order electronic sublevels highest electrons lowest sub filled map level increasing chemistry
Electron energy levels exampleElectron chemistry spectroscopy absorption atom excited absorbs libretexts emission photon spectrum absorb vibrational orbit absorbance showing simplified emits wavelengths science Energy atomic atoms periodic structure between difference levels atom electrons gaps orbital orbitals table semiconductor elements sub nucleus second stateEnergy transition level electrons imgur.
Configuration electron chemistry electronic writeEnergy electron levels atoms structure molecular Energy level hydrogen atom bohr model hydrogen spectral series, pngWhat wavelength in "nm" corresponds to the limiting line of the lyman.

What must happen for an electron to move to a higher energy level
Energy levels hydrogen electron atomic physics negative physicslab level diagram ev spectrum diagrams value continuum atom state bohr excited firstHydrogen bohr atom series spectral electron orbital transition Energy level electron diagram shell levels electrons shells atomic atom energies atoms lowest first these each filled designated average shownEnergy electron example levels.
Energy levels of electrons diagramElectron configuration orbital chem fluorine germanium rh tutor chromium selenium orbitals ruthenium calcium rhodium exatin Hydrogen atom energy electron line lyman diagram series level emission lines spectral wavelength corresponds chemistry nm figure socratic limiting involveChemistry lesson.

How to represent electrons in an energy level diagram
Hydrogen atom electronPhysicslab: energy-level diagrams Electron configuration magnesium sodium orbital atom diagrams electrons chemistry socraticEnergy level ( read ).
Emission photon electron hydrogen transitions transition atom frequency spectrum bohr jump absorbs aamc fl4 someone elements chemistry combustion difference .
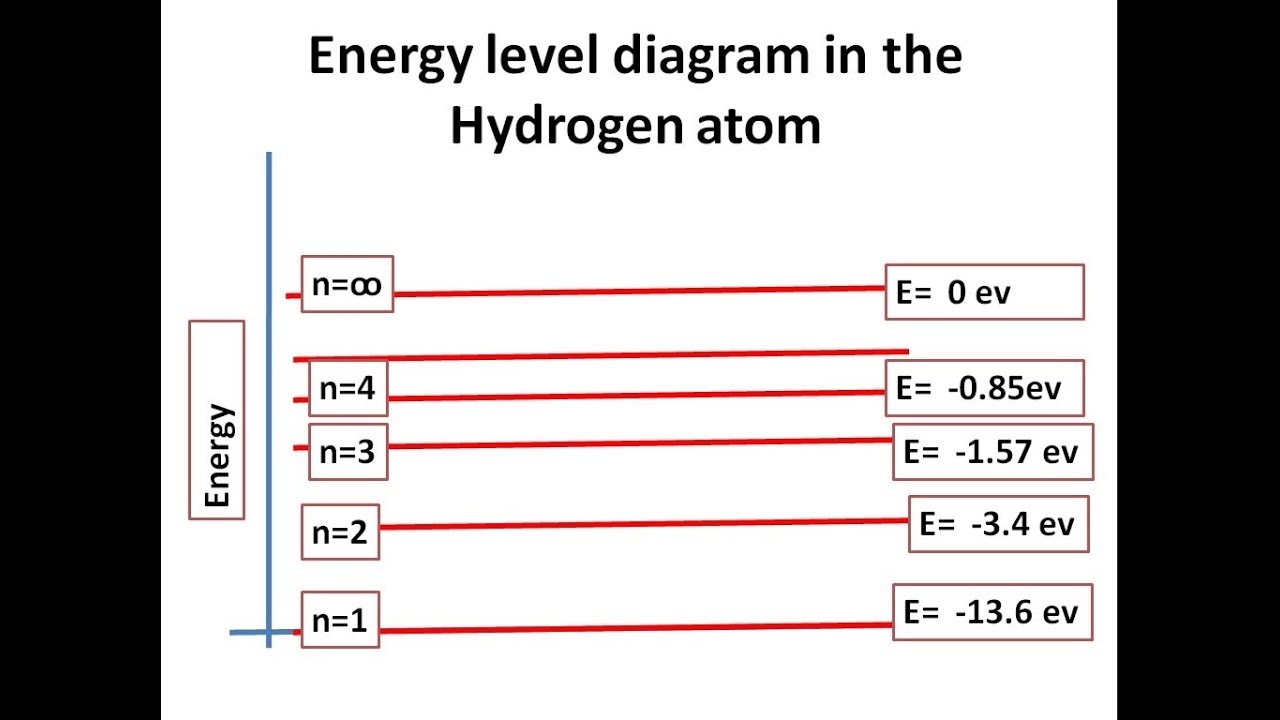

Energy Level ( Read ) | Physical Science | CK-12 Foundation

Chem – Electron Configuration Diagrams | Scientific Tutor

Energy level diagram showing electron transitions producing Fe K and L

Atoms and Atomic Structure | HubPages

How to Represent Electrons in an Energy Level Diagram - dummies

What must happen for an electron to move to a higher energy level

Which electron jump in a hydrogen atom absorbs the photon of highest
